Hello and welcome to a new post. Today I show you 3 simple methods for plastics identification.
There are several situations in which knowledge about the polymer you are handling is of interest. This can be quality issues or if parts failed you would like to know what polymer was involved.
Method nr. 1: Check solubility of polymer in solvents
There are six common solvents which allow you to identify the involved polymer due to solubility:
- Water: Polyvinyl alcohol (PVA), Polyethylene oxide, Polyacrylic acid
- Tetrahydrofuran (THF): all non-crosslinked plastics, except Polyolefines, Polyfluoro hydrocarbons, Polyacrylamide, Polyoxymethylene, Polyamides, Polyurethanes, Polyethylene terephthalate
- Xylene (Dimethylbenzene): Polyoelfins, Styrol polymers, Vinylchloride polymers, Polyacrylicester, Polytrifluoroethylene
- Dimethylformamide (DMF): Polyacrylnitrile, Polyformaldehyde
- Formic acid: Polyamides, Polyvinyl derivates
- Nitrobenzene: Polyethylene terephthalate
Method nr. 2: Check the density
A fast way to estimate the density (= mass/volume [g/cm3]) is by making a sink-float density measurement. In this test you bring your sample in contact with different fluids:
- Methanol (density = 0.79 g/ cm3 @ 20°C)
- Water (density = 1.00 g/ cm3)
- Magnesium chloride solution (density = 1.34 g/ cm3)
- Zinc chloride solution (density = 2.01 g/ cm3)
This allows you to get an opinion whether it is a high, medium or low density polymer.
In Table 1, several densities of unfilled polymers are listed. Additives such as glass fibers are impacting the density and this can be seen in Table 1 too.
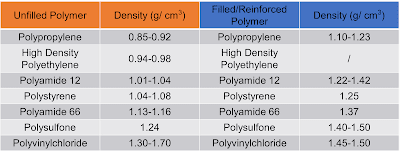 |
| Table 1: Comparison of the density of unfilled and filled polymers |
Method nr. 3: Check behavior due to heat exposure
In the third method we want to obtain the degradation result of our polymers when they are heated above the melting temperature. Low molecular weight side products will burn too and have a characteristic smell. For this we put a small amount of sample (100 mg is enough) into an ignition glass tube. Then we place a pH-paper or Litmus paper on the open end and start heating the sample over a Bunsen burner. The degradation vapor will react with the paper and we obtain red color (acid), neutral (no color change), or blue color (base/alkali).
Following polymers are linked to the different colors:
- Red / pH 0.5-4.0: PVC, PET, Fluoropolymers, Polyvinylesters
- Unchanged / pH 5.0-5.5: Polyolefins, PVA, PS, POM, PC, Silicones, Phenolic- and Epoxy resins
- Blue / pH 8.0-9.5: PA, ABS, PAN, Melamine resins
Also, in the following I summarized the aforementioned 3 methods in an infographic:
 |
| Plastics Identification - 3 Simple Methods |
Furthermore, I made training videos with more details on the different methods:
Part 1: 3 simple methods
Part 2: identify polyolefins, polyamides, polycarbonates and high heat plastics
Part 3: identify plastic compounds with IR-spectroscopy
Thanks for reading!
Greetings and #findoutaboutplastics
Herwig Juster
#plasticsidentification #findoutaboutplastics
Interested to talk with me about your plastic selection and part design needs - here you can contact me


JackpotCity Casino Site - LuckyClub
ReplyDeleteJackpotCity Casino - The World's Favourite luckyclub Online Casino with Sports Betting, Slot Games, Keno, Poker, Roulette and a Full-Service Casino. JackpotCity is your